strong nonspecific in EMSA - (Nov/05/2011 )
hi I wanted to check if protein A affects DNA binding ability of protein B, a transcriptional factor, so I performed EMSA. The photo was attached. I have several questions.
1. There are at least 2 non-specific bands which remained there after the addition of unlabeled probe (lane 5,6). However, lysate 1 (lane 1,4,7,10) without protein B has no such non-specific bands.
How can I remove those?
2. There seem to be 2 shift (lane 2,3) but this FLAG-tagged protein B only has one transcript, according to my western blot result. How to explain this?
3. 2 supershift were observed (lane 8,9) using antibody to B, but they are not very clear. How can I improve this?
4. My immunoprecipitation result suggests that A binds to B, but why can't I get a supershift by using antibody to A (lane 11, 12)?
5. What are those remained in the loading well?
I used the kit from Roche called "DIG Gel Shift Kit, 2nd generation". For supershift, 3 ul of anti-FLAG or anti-Myc were added to the reaction and incubated on ice for 1 h. For all the reactions, I added the labeled probe last trying to lower the background. And after incubation at RT for 15 min, I loaded the samples to 8% TBE gel (Novax).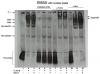
by the way, is there a method to strip the anti-DIG in the Nylon membrane so that I can reprobe with some antibodies to check if the shifted bands are specific?
