How do I get rid of this contaminant? - His-tagged protein eluted with 300mM imidazole (Aug/17/2009 )
Hello!
I have tried and tried to get rid of this high MW contaminant. I have 40mM imidazole in the binding buffer. I wash with 60 mM and 100mM in 300mM salt buffer, I elute with 300mM imidazole. How can I get rid of this band?? I need the protein for antibody! The buffers are pH 8 btw.
Any suggestions or tips? I attached a lil pic of my elution fraction just so you can have a better idea of what im talking about
Thank you in advance for any comments!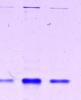
Hey,
Are you sure its a contaminating protein? If it stuck at the bottom of the well, then it could be an artifact. Sorry but I can't make out.
If it is indeed a contamination, I doubt if it would go. You can try:
1. Increase salt to 500 mM
2. Try FPLC
If nothing works, cut the band and electroelute.
Best,
TC
PhD on Aug 17 2009, 07:47 PM said:
I have tried and tried to get rid of this high MW contaminant. I have 40mM imidazole in the binding buffer. I wash with 60 mM and 100mM in 300mM salt buffer, I elute with 300mM imidazole. How can I get rid of this band?? I need the protein for antibody! The buffers are pH 8 btw.
Any suggestions or tips? I attached a lil pic of my elution fraction just so you can have a better idea of what im talking about
Thank you in advance for any comments!
Hi!
Thanx for the reply! what do u mean electrolute? I thought maybe there was another column I could pass it through maybe?
What's the MW for your protein? Maybe you can try if ultrafiltration membrane with MW cut off greater than your protein to filter off the high MW stuff? You can get these as centrifugation units from Amicon in various capacity and selection of MW cut off.
Hi!
what do you mean by ultrafiltration? My fragment is around 14kDa so I could use a cutoff that is like 20 or so and my protein would flow through and the contaminants wouldnt?? Is it the same as dialysis? Could I try doing dialysis with 3ml elution fragments and 2ml dialysis buffer and let my protein flow through into dialysis buffer while the contaminant stays?? Sounds intriguing and cool!!
No, it is a membrane with pores. The membrane is mounted to a V shaped device and placed on top of a centrifuge tube . you load your sample to it and spin at 4000 rpm at 4 C for 30 min to 2 hr. If your fragment does not form dimer or something, you may try the one with 30k MWCO.
However, it could be, just like TC has said, an artifact.
Ha! I looked around and found amicon ultra centrifugal filter devices with a 30kDa cutoff!! I will try this!
Also, you can try another chromatographic step, for example IEX at a specific pH, sometimes the pI is different enough to discriminate both proteins. If you are producing your recombinant protein in E. coli, most of the bacteria proteins are acidic, maybe you can calculate the theorical pI of your recombinant protein. If this is a basic protein, pI>7, then you can separate both proteins by using a Q-HP or S-HP protein at a particular pH which discriminate the binding of one of your proteins. Maybe you can use this step as a capture one, before Ni or as a polishing, after Ni to get rid of this high molecular weight band.
Good luck
IEX or HIC would help separating your protein from high MW contaminant.
I would start with IEC>HIC then go for SEC. So, let's try IEC first but check the pI of your protein first. You wouldn't want precipitated protein. : )
Are you sure you want to immunisemice with His-tagged protein?? His tag is known to be highly immunodominant and you might end up generating more antibodies against the tag than the protein itself.
