abnormal band in agarose gel - Urgent help required (Jun/22/2009 )
Hi.
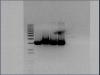
I have a problem with my agarose electrophoresis gels. This is the second time this is happening. The bands appear to be formed by small blebs or clusters. Very abnormal shape. I've never seen this before. I am not sure what the cause may be.
I get this problem when running a digestion of plasmid DNA, having an insert, on the gel. I use ultra-pure agarose, so that I can excise the digest fragment from the gel for ligation and for other cloning purposes. I make a 0.8% gel, and the size of my insert is 2.6kb, and vector is 2.9kb (pbluescript). I put 7 or 8 ul of plasmid DNA for digestion, and the concentration of DNA ranges from 200 to 370 ng/ul. Could it be because I put too much DNA in the digestion? (either because of high conc., or because I am running the whole 40ul of digestion in a single well?)
I have attached the pictures of the gel so that you can see what I'm talking about. Both pictures are of the same gel.
Chill, its just too much DNA per lane!
Wait until you have a real problem....gonna blow a gasket.
vvslab on Jun 23 2009, 06:35 AM said:
I have a problem with my agarose electrophoresis gels. This is the second time this is happening. The bands appear to be formed by small blebs or clusters. Very abnormal shape. I've never seen this before. I am not sure what the cause may be.
I get this problem when running a digestion of plasmid DNA, having an insert, on the gel. I use ultra-pure agarose, so that I can excise the digest fragment from the gel for ligation and for other cloning purposes. I make a 0.8% gel, and the size of my insert is 2.6kb, and vector is 2.9kb (pbluescript). I put 7 or 8 ul of plasmid DNA for digestion, and the concentration of DNA ranges from 200 to 370 ng/ul. Could it be because I put too much DNA in the digestion? (either because of high conc., or because I am running the whole 40ul of digestion in a single well?)
I have attached the pictures of the gel so that you can see what I'm talking about. Both pictures are of the same gel.
The bands are due to the high amount of DNA. Plus, the size of your insert and the vector is pretty near; they only differ by 300bp. After digestion, there will be your insert and your vector in the solution. When the amount of DNA is high, you can get blebs like what you see.
Well, at least you got what you want.
