strange extra bands on digest of miniprep? - (May/23/2009 )
Hi guys,
So I'm new to cloning, and I just completed a double digest with BamHI and XhoI of plasmids I purified from Qiagen miniprep. The vector I'm using is about 5 kb, the insert 1 kb. After a two hour digestion, I get the following result (attached).
I was thinking, if my digest was complete, I would get a 5 kb empty vector band (which corresponds with the top row on my gel) as well as a 1 kb insert band (in some of my preps). But why is there a 4 kb band (second row) across the board?
I guess my question is, why is there the presence of a 4 kb band in all of my minipreps, regardless of whether or not my original vector had the insert or not?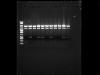
Not sure but these could be possible reasons:
1. Uncut supercoiled form of DNA. Digest less of DNA and check.
2. Enzyme vial is contaminated.
3. Contamination in the minipreps.
Seraph524 on May 23 2009, 11:31 AM said:
So I'm new to cloning, and I just completed a double digest with BamHI and XhoI of plasmids I purified from Qiagen miniprep. The vector I'm using is about 5 kb, the insert 1 kb. After a two hour digestion, I get the following result (attached).
I was thinking, if my digest was complete, I would get a 5 kb empty vector band (which corresponds with the top row on my gel) as well as a 1 kb insert band (in some of my preps). But why is there a 4 kb band (second row) across the board?
I guess my question is, why is there the presence of a 4 kb band in all of my minipreps, regardless of whether or not my original vector had the insert or not?
Undigested supercoiled plasmid should be the one thing i will put my bet on. Double digest usually gives this sort of thing . Sometimes bad plasmid prep introduce contaminant which further wreck the digestion to be incomplete. Try prolonging the digestion or adding more RE. see if that works.
Thanks for all the help guys!
My PI told me that it was simply supercoiled DNA and that its not abnormal to see them in rest. digests of plasmid preps.
So the posters/members above were right on the dot!
Thanks!
Hi!
Always load undigested vector next to your digested samples. This gives you the possibility to check whether the unwanted band is uncut vector. Good luck!
isbow on May 25 2009, 06:17 AM said:
Always load undigested vector next to your digested samples. This gives you the possibility to check whether the unwanted band is uncut vector. Good luck!
Will do next time! Thanks!
