TMRE histograms - need advice please (Apr/01/2009 )
Dear All
I have used TMRE to measure mitochondrial membrane potential for SW480 cancer cells treated with an agent which induces apoptosis after 24 hrs of exposure. I aimed to measure the mitochondrial membrane potential after 12 hrs of exposure, where I collected the cells by trypsinization, and then loaded them with 100nM TMRE for 10 minutes at 37 oC then kept them on ice and immediately analysed them within 1 hr. I aquired 20,000 cells and did not use a gate. FSC threshold was set to 52. As demonstrated on the histograms in the attached JPEG file, following drug treatment, there is an increase in population (I) and a reduction in population (II), as compared to control, while I was expecting the opposite. Actually I can not explain this reproducible result and I greatly appreciate your responses.
thank you very much.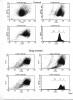
Hey yobou
Did you manage to figure out the reason? I have a similar problem at the moment and would be grateful for advice.
Now I load the cells while adherent first with TMRE/PBS for 30 min at incubator then harvest with trypsin and wash once with warm PBS then immediately analyze at RT
And now your results are like they should be? So you see a decrease in TMRE signal after treatment with pro-apoptotic agents in comparison with untreated cells?
yes
Sorry, one more question. When you add the TMRE, do you still use 100 nM concentration? I'm asking it because according to my protocol the concentration should be 40 nM, but this is for 1 million trypsinised cells, so it might be too low.
I use 100nM
I just tried it out and it worked perfect. Thanks a lot for your advice!
you are welcome
