Sequential restriction digestion of plasmid - (Feb/21/2009 )
Thanks TC, can u show me what is the ratio u used for restriction digest?
Normally I will put
7ul of ddH2O
1ul 10x buffer
1ul sample
1ul of Enzym (EcoRI or BamHI)
my sample concentration were around 1.8-2.0 ug/ml
will it be too little??
Please let me know your sample preparation please.
and can u see clear fragmets when digested??
Thank you very much
T C on Feb 24 2009, 05:13 PM said:
Maybe the DNA you are taking for setting up digestions is low in concentration, digest more of it.
Best
TC
Just curious question...
during all of your digestion...can you all see the abvious fragments on the gel ??
I couldn't view the clear fragments from the gel ...but using lamda DNA I can see the clear fragments.
I am using enzyme EcoRI and BamH1..
Thank you
Hey
We do a lot of cloning in the lab so don't really quantitate the DNA before digestions. I normally grow a 3ml culture for 12 hrs and do a miniprep without using the column and resuspend in 30ul water.
Take 0.5 ul of this DNA, 2ul NEB buffer(1X), 0.3 ul enzyme, 0.2 ul BSA(if required)(1X) and rest is water in a 20ul digest. Digest 8-13 hrs for a prep digestion and put up multiple digestions if you want more of the digested product..
I see a very distinct band on the gel.
Let me know if you want to know anything else.
Best
TC
jiajia1987 on Feb 24 2009, 05:53 PM said:
Lightly vortexed. Just touched the tube.
perneseblue on Feb 25 2009, 05:52 PM said:
jiajia1987 on Feb 24 2009, 05:53 PM said:
Lightly vortexed. Just touched the tube.
Yep! will do that in future! thanks heaps!
Just curious...
So after linearinzing the plasmid by enz digest, is it going to exist in super-coiled form or just circular DNA? Or The plasmid becomes super-coiled only after transformation because when I miniprep, it is in super-coiled form.
Thanks TC for ur reply, I normally use specific buffer for the enzyme ..My enzyme is from Promega, will it be less efficient??
If you don't mind can u send me ur gel photo which contain digested DNA??
THank you very much.
T C on Feb 25 2009, 04:07 PM said:
We do a lot of cloning in the lab so don't really quantitate the DNA before digestions. I normally grow a 3ml culture for 12 hrs and do a miniprep without using the column and resuspend in 30ul water.
Take 0.5 ul of this DNA, 2ul NEB buffer(1X), 0.3 ul enzyme, 0.2 ul BSA(if required)(1X) and rest is water in a 20ul digest. Digest 8-13 hrs for a prep digestion and put up multiple digestions if you want more of the digested product..
I see a very distinct band on the gel.
Let me know if you want to know anything else.
Best
TC
Hey
I don't think the company makes a difference as long as you stick to teh protocols. Here is a gel pic of one of the clones digested with Nde I/ Hind III. But I wonder how this would help. ![]()
Lane 1,2,3: digestion in triplets
4: 1 kb ladder
5: 100 bp ladder
Best
TC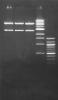
Thanks for your prompt reply.
It is helpful by looking at the photo because I know that your DNA size are less than 1Kb...
I have DNA that are very big..more than 1kb at least...
have u try digest DNA that have more thab 1kb ???
Would u still see the distinct band for DNA more than 1kb??
Thanks TC
T C on Feb 26 2009, 04:55 PM said:
I don't think the company makes a difference as long as you stick to teh protocols. Here is a gel pic of one of the clones digested with Nde I/ Hind III. But I wonder how this would help.
Lane 1,2,3: digestion in triplets
4: 1 kb ladder
5: 100 bp ladder
Best
TC
Hey
My mistake....should have read the ladder for you
Read it this way
Lane 1,2,3: digestion in triplets
4: 1 kb ladder, from top read it this way: 10kb, 8kb, 6kb, 5kb, 4kb, 3kb, 2kb, 1.5kb, 1kb, 500bp
5: 100 bp ladder, from top read it this way: 1.5kb, 1.2kb, 1kb, 900bp, 800bp, 700bp, 600bp, 500bp, 400bp, 300bp, 200bp, 100bp.
So the bands are ~2.8 and ~5.5 kb (can dig for the exact sizes if u want).
Best
TC
Thanks for your prompt reply.
It is helpful by looking at the photo because I know that your DNA size are less than 1Kb...
I have DNA that are very big..more than 1kb at least...
have u try digest DNA that have more thab 1kb ???
Would u still see the distinct band for DNA more than 1kb??
Thanks TC
oh..
Thanks again.
TC, later I will attach my photo which I digested with EcoRI and please advice me
I am digesting Banana DNA..
Thanks
