Getting terrible grainy background in immunofluorescence experiment - (Jan/03/2017 )
Dear all,
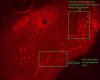
I am doing immunofluorescent staining in mouse embryonic brainstem tissue against many different transcription factors. We use 30 min 4% PFA fixed cryosections of ca 14 um thickness. All of my antibodies that are not produced in rabbit gives beautiful staning with minimal background or unspecific staining. However, with all of my rabbit primaries I get unspecific staining all over the tissue which looks like very small particles (grains/dots). With a confocal microscope I see that they are in the whole depth of the tissue, but more so on the surface of my sections. This happens with 4 different secondary antibodies that I have tried. I have tried using both donkey and goat serum for blocking with the same result for both. Also, the serum, PBS/TBST used for washing, and mounting media should be fine as there is no unspecific staining seen with the same reagents in non-rabbit immunos. See the two attached images for examples of the grainy background. It looks even works in confocal images.
I have tried spinning down the secondary in pure TBST for 30 min and use only the supernatant but it doesnt work. Negative controls without primary antibody appears to have less grains, but I think that might just be due to slightly higher overall background that masks some of the grains.
Anyone has any ideas or experience with this kind of problem?
Best regards,
Anders
P.S.
I have posted this question on ResearchGate as well, but I didnt get an answer for anybody who had specifically dealt with this phenomena before (grains/dots). I therefore hope someone here can tell me exactly what this is! See full researchgate discussion below:

If this happens only with rabbit antibodies, then the problem won't be with the secondaries, unless they are rabbit-raised too.
Occasionally you get primaries that clump/precipitate and cause this sort of signal. I see you tried it for the secondaries, but did you also try spinning down the primary (note: usually brief spins of 1 min or so at 16 000 rcf work for this)? I would do this on the antibody tube itself, not the dilution.
Another thing you could try is to use a different colour secondary and see if the signal goes away.
Negative controls without primary antibody appears to have less grains, but I think that might just be due to slightly higher overall background that masks some of the grains.
This quote is inconsistent with the first part of your post, first you say that with the non-rabbit Abs you get good clean images, but here you say that in the absence of any primary you still have the problem... could it be that you have a problem with a batch of sections. Could you put a section under the microscope with no primary or secondary? This sort of signal is sometimes seen as paraformaldehyde autofluorescence, but that normally occurs in the green/yellow spectrum, not the red. Try washing a section in 0.1M glycine/PBS overnight and see if the signal goes away.
Yeah, my simple negative controls were a bit inconclusive at first but now I see in some new experiments that they dont give any background or unwanted signal at all. There is a section batch effect, but the unwanted signal still persists across all batches. I like the idea of spinning down primaries, but wouldnt it be better to do in the dilluted solution? I have tried a Alexa488 secondary, which appears to help a little bit, but now the real signal is quite much weaker, and some grains still persist. It's really puzzeling. Bottom line is goat, mouse, guinea pig gives beautiful staining in the same sections but with 2 different rabbit antibodies I get this really characteristic grainy/particulate background staining, increased background, and usually poor main signal.
I like the idea of spinning down primaries, but wouldnt it be better to do in the dilluted solution?
No - the clumps of antibody are then transferred to your secondary and when you spin this down you loose much of the staining you might otherwise have had. You need to pellet them in the stock solution as the concentration of antibody is much higher so you still get approximately the same concentration as before the spin, which can then be used to make your dilution in the absence of clumps.
Just a thought - do you use the same (red) channel for imaging with the other antibodies? If not it may just be an apparent lack of spots, red signals tend to be weaker (visually and in reality) than the corresponding greens and blues, so we tend to compensate by using more power to excite the fluorophore, which then leads to higher background. It might be that these spots are there for the other color sections, just not readily seen as you have a greater signal from the secondary for the same amount of light.
Hi again, and thanks for your posts! I always spin the stock before taking, but I'm not so careful to only take the top volume. Maybe this is a key factor. It's only a 20uL stock, so I easily reach the bottom of it. I also spin just ~5 seconds on low centrifugal force, so I'll increase it next time. However, when run in parallel I saw that tissue batch is important, as much less grains was seen in one prep than another, using the same antibody working solution (I've run different tissue batches in prallel, using otherwise same conditions, with a really marked difference).
bob1 on Wed Jan 4 14:55:57 2017 said:
I like the idea of spinning down primaries, but wouldnt it be better to do in the dilluted solution?
No - the clumps of antibody are then transferred to your secondary and when you spin this down you loose much of the staining you might otherwise have had.
You might misunderstand me? I meant to spin down a working solution of primary antibody, and carefully use only the top ~80% of the volume. This should leave any precipitated antibodies behind, right? As my most important rabbit primary is only a 20uL test aliquot from Bethyl (cMaf) taking the only top volum is a bit more tricky (poor transparency of bottle).
I always use the same imaging/visualizing setup for "red" channel, across different antibodies. For example I used a goat-antibody1 and a rabbit-antibody2 in a parallell experiments, label both with Cy3-secondaries, and the goat one is beautiful with 0 background, while the rabbit is really bad. And like I said, the bad rabbit staining happens with 4 different secondaries I've tried. The problem is also very apparent in at least 3 of 4 rabbit primaries I have, but almost never seen in 10-20 other primaries I use from different species.
After reviewing all my tests and controls it is clear to me that this phenomena is sensitive to tissue batches. I try to treat each tissue preparation similar, so it must be rather sensitive. FYI it is embryonic mouse or chicken brainstem preparations that are labeled with fluorescine-dextran and incubated in ACSF for ~9 hours, before 30 min 4% PFA fix in cold, 20-30% sucrose treated, OCT embedded and cryosectioning. Storage if OCT blocks, cryosections, ACSF composition are possible important parameters.
I was a bit unclear before, but multiple tests show that my controls which exclude primary antibody never gives any background.
My only hope now is to make a few new batches of tissues, and hope they will be better. Also possibly take more care with the spinning of primaries.
If that's what you meant, then you are doing it correctly. I would, as you suggested, do a longer spin.
If your staining only happens with the rabbit primaries, then it is definitely a primary issue in conjunction with a batch issue. Perhaps there is something going on with your fixative and it isn't working as well as it might.
Your protocol for fixing looks fine, but I've not done any brain work and little on cryosections so I'm no expert there. I would however try to get rid of as much of the PFA as possible (wash wash wash), and make sure that you are properly dissolving it first . You may want to consider switching to formalin, but the methanol component can shrink tissues a bit, which may interfere with what you want.
