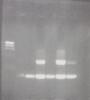
colony pcr, two strong bands - (Mar/27/2015 )
Hi! After sucessfully cloning my gene into the TOPO vector I performed up/down pcr (inverse pcr) and ligated with a new insert we use for gene deletions in our lab. After transforming onto antibiotic selection plates I proceeded to screen those colonies with colony pcr. I see bands of expected size with my new insert, but also a second band of equal intensity that appears in both insert positive and negative cases. However this second band is not the proper size to be self-ligation. If anything it is possibly the same size as the original insert. If it is, how can I have both? I will try adding an image later, but if anyone has any ideas let me know! Thanks
I would try re-streaking your colonies on a new plate prior to colony pcr. If you have high concentration of DNA going into a ligation reaction, the transformation reaction can leave some of that DNA on the plate when you plate out the transformation. This can be picked up in the colony pcr, and yield a band. You could also have a mixed colony, with some of the original plasmid transforming. In either case, restreaking will solve the problem.
phage434 on Sat Mar 28 12:53:33 2015 said:
I would try re-streaking your colonies on a new plate prior to colony pcr. If you have high concentration of DNA going into a ligation reaction, the transformation reaction can leave some of that DNA on the plate when you plate out the transformation. This can be picked up in the colony pcr, and yield a band. You could also have a mixed colony, with some of the original plasmid transforming. In either case, restreaking will solve the problem.
Thanks! Unfortunately, this problem persisted after restreaking. That was my first thought as well...
Here's the image, the two larger bands ~3kb are the expected bands. Ladder is lambda hindIII. This was after re-streaking.