Ligation: is it possible to ligate two vector backbones together - (Aug/25/2014 )
Greetings
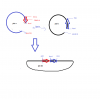
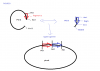
So I generated two fragments (A & B ) using PCR and using TA cloning I inserted the fragments into pTZ57R/T plasmids to generate plasmids: ptz-a and ptz-b.I would then, use asc 1 and hind 3 enzymes ( as in attached rough sketch image) to cut out each fragment and then ligate them together to form ptz ab, so digestion of ptz a would be 3220 bp and include the fragment a, while digestion of ptz b would only be 249 bp and include fragment b. so i would ligate the two hind 3 will ligate to hind 3 and asc 1 to asc 1 ultimately putting fragment a and b together ( FIGURE 2). So basically after digestion from ptz b with hind 3 and asc 1 i would extract the 249 bp fragment and from the digestion of ptz a i would use a 3220 bp fragment ligate the two n get ptz ab. however my ptz b plasmid has the insert in the reverse orientation ( figure 3). so my supervisor said I would have to search for new enzymes to cut ptz b . He was saying this because if i had kept to the same plan as before the fragment from ptz b that i would need to ligate to the fragment of ptz b, would now be 3051 bp instead of 249 bp. so ultimately it would mean fusing 3051 bp to 3220 bp. is this possible? or does ligation always need to have a very small insert? or is he saying this because somehow if its in reverse orientation it changes reading frame or something. im so confused.
sorry for the bad image its just a rough sketch
Your supervisor is correct.
It is possible to ligate fragments of "any" size together. Normally when you create constructs of this sort, you want both insert ORFs to run in the same direction, so that the promoter can initiate transcription of the insert. If you want a fusion protein, then you need them to be in-frame as well. If you want separate transcription you can use an IRES sequence. One easy way to do this is to design primers for your sequence that contain restriction sites at either end for the REs you want to use to clone the insert. You can then digest the PCR product and the plasmid and ligate.
Note that according to your map, digestion with AscI and PciI will cut out your insert A when you do the digestion. If you want both inserts in the same plasmid, you need to use 2 REs (or just one, if you don't care about orientation), that are on the same side of the insert you want to keep.
@bob1 Thanks for your reply. i noted i may have listed the wrong enzymes in my question but i edited it right now and added more description and added new images. thanks
bob1 on Mon Aug 25 10:01:27 2014 said:
Your supervisor is correct.
It is possible to ligate fragments of "any" size together. Normally when you create constructs of this sort, you want both insert ORFs to run in the same direction, so that the promoter can initiate transcription of the insert. If you want a fusion protein, then you need them to be in-frame as well. If you want separate transcription you can use an IRES sequence. One easy way to do this is to design primers for your sequence that contain restriction sites at either end for the REs you want to use to clone the insert. You can then digest the PCR product and the plasmid and ligate.
Note that according to your map, digestion with AscI and PciI will cut out your insert A when you do the digestion. If you want both inserts in the same plasmid, you need to use 2 REs (or just one, if you don't care about orientation), that are on the same side of the insert you want to keep.
@bob1 Thanks for your reply. i noted i may have listed the wrong enzymes in my question but i edited it right now and added more description and added new images. thanks
While you can ligate any pieces of DNA together with compatible ends, usually circular DNA fragments with more than one origin of replication will fail to transform. This is helpful, as it avoids plasmids which form from concatamers, which ligation can easily produce.
bob1 on Mon Aug 25 10:01:27 2014 said:
Your supervisor is correct.
It is possible to ligate fragments of "any" size together. Normally when you create constructs of this sort, you want both insert ORFs to run in the same direction, so that the promoter can initiate transcription of the insert. If you want a fusion protein, then you need them to be in-frame as well. If you want separate transcription you can use an IRES sequence. One easy way to do this is to design primers for your sequence that contain restriction sites at either end for the REs you want to use to clone the insert. You can then digest the PCR product and the plasmid and ligate.
Note that according to your map, digestion with AscI and PciI will cut out your insert A when you do the digestion. If you want both inserts in the same plasmid, you need to use 2 REs (or just one, if you don't care about orientation), that are on the same side of the insert you want to keep.
IRES are related to translation, not transcription.
https://en.wikipedia.org/wiki/Internal_ribosome_entry_site
Raygoza on Mon Aug 25 20:12:58 2014 said:
bob1 on Mon Aug 25 10:01:27 2014 said:
If you want separate transcription you can use an IRES sequence.
IRES are related to translation, not transcription.
https://en.wikipedia.org/wiki/Internal_ribosome_entry_site
Whoops, yeah. was initially writing something about transcription, then changed it...
How to purify the PCR product after enzymatic digestion before ligation reaction?
heat inactivate and put it on a column (like a PCR clean up)
its just one option.
Some people even put it on a gel, but I would not recommend that, since you lose so much DNA.
I used to use DNAbeads in my previous lab (Magnetic beads). do you think ethanol perception is ideally better in this case?
