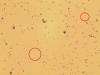
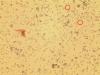
Caco-2 cells with unidentified contaminant - (Aug/14/2014 )
Hello everyone,
I am new to this forum, but it looked like a helpful place to get other's opinions! Please let me know if further information is required.
I am a technician doing some work with Caco-2 cells, and my lab is currently having issues maintaining our cell line. A few notes
- Cells are obtained from ATCC
- Media is M199 with the addition of streptomycin/penicillin (1%), non essential amino acids (1%) and FBS (NZ sourced) (10%)- a media which has been tested for suitability with Caco-2 cells.
- The cells grew well, and were passaged and frozen down successfully over the past 5 or so years, with new vials being brought up frequently for experments (where they are seeded first into flasks, bulked up, then seeded onto inserts)
It was noted that over the last 2 years or so that the cells have not been performing as they had been, with low TER, resistance to passaging (very sticky), and cell clumping following a split (using Trypsin initially, and later we switched to Tryple express).
Within the last few months it has come to our attention that there are smaller cells visable when the Caco-2s have been visualised and counted using trypan blue and a countess machine for cell counting- and also using a haemocytometer. These smaller cells are now visible under 40x magnification inside our flasks also (and they appear to be adhering to the flask, not other cells), and are more prolific. The countess has told us that these cells are less than 20um.
I have attached 2 photos, the first being the cells in the antibiotic media, and the second being cells following a switch to antibiotic free media. If anyone can shed any light on why we might be having these issues it would sure be helpful! The smaller cells are circled in red. These photos were taken from a cell suspension.
Thanks.
Edited to add: Media is holding pH as normal and does not appear turbid. All recommended aeseptic technique is adhered to :-)
It does look like a contaminant, there are too many and they are too regular to be cellular debris. However, just to make sure, can you get hold of some DNA stain such as DAPI or Hoescht33258 and do a quick stain - fix, permeabilize, DAPI stain, wash, mount and view under fluorescence - to confirm that these contain DNA.
There is a reasonable chance that these are a sort of yeast, they are too big for most forms of bacteria, but I couldn't see an of the characteristic clumping.
Generally, the best idea is to get rid of anything suspect - would you trust any results you got from them? I would go back make sure you got fresh everything (buy some commercially prepared medium if you don't already), did a really good clean of the lab and equipment, and then thaw a very early frozen stock to see if that gets rid of the problem.
Thanks for the ideas!
The clumping occured while we were using Tryple express. It stopped working efficiently so we switched back to trypsin, which gave us this nice single cell suspension for the first time in months. But, the second time we tried to split with tyrpsin we got clumpy cells again (sorry I don't think I have a picture), and the cells refused to dislodge from the flask at all without force- at 60-70% confluence.
We use all commercially prepared media, and use UV lighting at the end of each day.
Late last year it was suspected we may have had yeast- but it may have been misdiagnosed (I believe agar test came back clean) but the lab was thoroughly decomissioned and decontaminated anyway- stocks were chucked out, bleached/autoclaved equipment, and even had a commercial decontamination unit in to fumigate.
These cells appear to be in stocks from many points in time from different users. We have ordered a new cryovial from ATCC which is due to arrive later this month (and the lab will be cleaned before this arrives), but we need to figure out what the cells are so that we can resolve the problem so it never occurs again!
They have been observed to exhibit brownian motion- for which yeast cells are too big right?
I will look into the staining next week to confirm DNA presence :-). I am not sure if anyone here has done the staining before so I shall ask around.
Oh and edited to add: yes, all experiments are currently on hold (since we noticed this issue) until it is sorted once more!