Low signal, weird background stains on blot - (Feb/04/2014 )
Hi all,
I am trying to detect a ~80kDa protein in HEK and Cos cell lysates. I use the purified GST-tagged protein as control and standards on the western blot. Last week, when I used 5 - 50ng of the purified protein for the controls, I got very clear bands. But this week when I ran the western blot again using the same proteins, I got very low signal. I am also getting some weird stains on the blot.
For the primary Ab, I use 1:200 and incubate overnight at 4 degree. For the HRP-conjugated secondary Ab, I use 1:6000 and incubate for 1 hour at RT. I rinse 4x 5-7min after each incubation, and use Pierce ECL kit and the LICOR system to visualize the blot.
I have attached one western blot from last week when it was fine, and one from this week where I could barely get any signal.
What could be causing this issue?
Thanks!

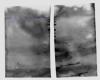
I would say you have a lot of background staining in both blots, which either indicates that you haven't blocked the membranes enough, or that your secondary is too concentrated and/or incubated for too long.
However, the swirly background you see is due to liquid swirling between the membrane and gel during transfer. Light bands on a dark background can indicate that there is too much protein present in that lane (essentially burnout of the HRP). Spots on the membrane can come from a number of sources, dirt, forcep marks, precipitated antibody, overlapping membranes, and nicks/holes and folds in the membrane can all cause these.
The reason you can't see your bands this time around could be a huge range of things - is the protein stable? Are the antibodies stable? Did it transfer properly (ponceau stain?)? Did you make your PBS-T/TBS-T properly? Did the membranes dry out...
Perhaps the protein is not stable. I do not know how stable the antibody is; I only recently purchased it. Is there a way to find out?
I used the same transfer procedure for both blots, and the same batch of TBS-T.
I don't think the membrane dried out, but would it not work if the membrane dried out at some point?
The datasheet is the best place to start for antibody stability - it may well say storage conditions. I know of a few primary antibodies that will only last a matter of a day or two in the fridge, whereas others may last years. You could strip the blot that worked and re-probe with the antibody to see if it is an antibody problem
Drying of the blot is not a problem usually, but can cause high background especially if dried before blocking.
Is it nitrocellulose or PVDF?
It is nitrocellulose.
I repeated the blot, used new stock of purified protein and it worked. But I am still getting high background.
