SDS-PAGE abnormalities in proteins near to front dye - (Jun/24/2013 )
Hi,
I want to get some helps from all of you...
I have done a lot of SDS-PAGE with "strange" migration of protein near to front dye.
In a normal condition, the smallest protein marker should travel a bit slower than the protein samples migration indicator (bromophenol blue), i get a nice result last time (as shown in normal.png) but after i have prepared a new solution of acrylamide, the condition coming weird.
1. Smallest MW of my protein marker is 10 kDa, it will travel along with the samples indicator in a line, causing the bands near to the marker is distorted and cant get a good result. Somemore, during the destasining process, 1cm broad line apart from the "indicator blue line" is easily washed off and become transparent, so it seems abnormal.
2. The other condition is the smallest MW protein will travel slower than protein marker bigger than it and causing 2 markers were combined between each other.
Is it something happen in the polymerisation process?
Or acylamide contamination?
I tried to change the solution again and again and it doesn't helps.
So, now i m frustrating doing SDS-PAGE and hope to get your advises before i proceed it blindly.
Thanksss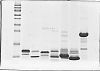
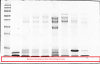
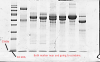
you'll note that you have a nearly transparent region in your "normal" gel as well as the third gel.
if you made the fresh acrylamide solution from powder then there may be some difference in concentration (weighing error, volume error).
and, the % crosslinker may be off for the same reasons.
there are other factors to consider. acrylamide, even in powder form, can decompose. your stock powder may be aging (or your old stock was and new stock can give you a different result.
the water you used to dissolve the powder (or dilute the concentrate) may be different (even from the same source, cartridges get used up, co2 dissolves in the water, oxygen dissolves in the water, etc).
contaminants in the acrylamide stocks may be different.
however, when buffer fronts appear to be a problem, the first thing i look at is the sds used in the buffers. did you prepare a fresh buffer with sds? how old was the sds stock?
try preparing fresh (with a different lot) sds solutions and buffers.
Hi mdfenko,
Thanks for your advice. It's really helps. I never though there is any problem in sds. I mixed sds inside my Tris buffer.
Thank you so much, i will try prepare sds freshly for the next sds-page and see how it goes.
Will update here very soon.
make sure you try a different batch of sds. the one you're using may be decomposed (enough to cause problems). if necessary, get it from a neighbor whose gels look normal.
Hi, i tried prepare new SDS and mix it when preparing gel. But the same condition still happened: the last marker flatten.
Somemore, i borrow commercial 30% bis/acrylamide from some other, i get the same result: the upper marker travel faster than the last.
I am frustrating why the things happened although i tried to change all the buffer and solution.
Please give me some advice regarding this.
Thank you so much.
the only thing left for me to suggest is for you to run the bromphenol blue to the end of the gel (or off the gel, we used to do this with gradient gels) to ensure that the buffer front passes the lowest marker. from what i saw in the original gels, the lowest proteins were still with the front.
can you show us the latest gels?
