Smear above plasmid dna - (Feb/26/2012 )
Hello.
In last step of Quiagen midi kit I ressuspend plasmid dna in 50 µl of ultrapure water.
After restrinction digestion with sacI and kpnI (fermentas) I run an electrophoresis and I get a smear above linearized vector.
Anyone know the reason why I get this smear. Maybe ultrapure water is contaminated.
I attached the agarosis gel eletrophoresis.
Thanks
Michael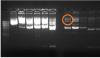
MW- Hind III marker
1- pQE30BG (beta giardin) uncut plasmid vetor
2- pQE30BG after 2 hours digestion with kpnI
3- pQE30BG after 4 hours digestion with kpnI
4- pQE30BG uncut plasmid vetor
5- pQE30BG after 2 hours digestion with sacI
6- pQE30BG after 4 hours digestion with sacI
7- empty
8- pQE30HBG after 2 hours digestion with kpnI
9- pQE30HBG after 4 hours digestion with kpnI
10- pQE30HBG uncut plasmid vetor
11- pQE30HBG after 2 hours digestion with sacI
12- pQE30HBG after 2 hours digestion with sacI
All have genomic dna above plasmid vector (blue rectangle)
In quiagen Troubleshooting Guide say that this is genomic dna contamination due to "Mixing of bacterial lysate was too vigorous. The lysate in the eluate must be handled gently after addition of Buffers P2 andP3 to prevent shearing of chromosomal DNA. Reduce culture volume if lysate is too viscous for gentle mixing."
But I´ve handled bacterial lysate gently after addition of Buffers P2 and P3 to prevent shearing of chromosomal DNA and reduced culture volume and still having genomic dna contamination.
Thanks.
Michael
I'd suggest that you are using too many cells in your prep. If you eluting a midiprep with 50 ul, you probably also have very high DNA concentrations. Your left gel is badly overloaded. Another problem is that the digestion with sacI and with kpnI yield different length fragments. Presumably they are supposed to be the same length. The extra DNA in the kpnI digest appears in the short band in the right most two lanes. Why is there such a disparity in the amount of DNA loaded in the different lanes?
Check you DNA concentration. I think the same that you are overloading the gels or using lot of DNA for restriction digestion. if it was genomic DNA you will always get a smear, but I see in the right wells, that you have discrete bands, which can only come from a plasmid b/c plasmid will yield only few bands and genomic DNA will yield so much bands that cannot be visualized seperately this way on gel.
