Tie-2 westerns high background black lanes - (Dec/15/2011 )
C:\Users\Archea\Downloads\13.jpgI am having a problem with Tie-2 western blots. There is only on strong band showed up around 250 kDa, whereas Tie-s should be 140 kDa. I also have high background and each lane shows strong signal. see attached picture.
Wet-transferred at 100V for 1.5h, with 10% Methanol, block with 5% dry milk in TBST for 1h; primary ab dilution, 1:500, overnight incubation @ 4。C; 2nd Ab, 1:10000 dilution, 1h @ R.T.
What is the problem that cause these black lanes? I used standard washing method: three times washing, each for 5-10mins.
Maybe it's the antibody problem. Try the same antibody from a different company. Some produce alot of non-specific binding. There seems to be a band at the 100-150kDa region in some of the lanes.
How long did you block the membrane in 5% milk? And what membrane were you using - nitrocellulose or PVDF?
science noob on Fri Dec 16 00:56:57 2011 said:
Maybe it's the antibody problem. Try the same antibody from a different company. Some produce alot of non-specific binding. There seems to be a band at the 100-150kDa region in some of the lanes.
How long did you block the membrane in 5% milk? And what membrane were you using - nitrocellulose or PVDF?
I used nitrocellulose membrane and blocked it for 1h @ RT.
Have you used housekeeping antibodies before? (tubulin, actin, GAPDH)
If yes, try to run one which you got nice staining from (low background). This can definitely confirm an antibody problem. Or try repeating the blot (same conditions) with another antibody which worked nicely prior.
you may want to try diluting the primary more. also, what is the diluent? you should dilute with blocking agent in the solution.
science noob on Fri Dec 16 07:53:35 2011 said:
Have you used housekeeping antibodies before? (tubulin, actin, GAPDH)
If yes, try to run one which you got nice staining from (low background). This can definitely confirm an antibody problem. Or try repeating the blot (same conditions) with another antibody which worked nicely prior.
Yes, I just tried actins and it turned out great. The 250kDa bands are still there, but they are weak.
mdfenko on Fri Dec 16 15:40:45 2011 said:
you may want to try diluting the primary more. also, what is the diluent? you should dilute with blocking agent in the solution.
I used 5% dry milk to dilute primary and secondary Ab.
science noob on Fri Dec 16 07:53:35 2011 said:
Have you used housekeeping antibodies before? (tubulin, actin, GAPDH)
If yes, try to run one which you got nice staining from (low background). This can definitely confirm an antibody problem. Or try repeating the blot (same conditions) with another antibody which worked nicely prior.
Attached picture is the b-actin film. What do you think? Can you be sure that tie-2 primary Ab caused this problem? Why are the 250 kDa bands still there? I stripped the membrane for 10min at least, but not over 15min, and then standard washing process.
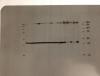
alex2815 on Fri Dec 16 19:51:06 2011 said:
science noob on Fri Dec 16 07:53:35 2011 said:
Have you used housekeeping antibodies before? (tubulin, actin, GAPDH)
If yes, try to run one which you got nice staining from (low background). This can definitely confirm an antibody problem. Or try repeating the blot (same conditions) with another antibody which worked nicely prior.
Attached picture is the b-actin film. What do you think? Can you be sure that tie-2 primary Ab caused this problem? Why are the 250 kDa bands still there? I stripped the membrane for 10min at least, but not over 15min, and then standard washing process.
So was this the same blot as the one you attached earlier post-stripping? The protein is definitely there and transferred well. But you need to make sure your Tie-2 is the correct size and not 250 kDa. Try running a few samples on a fresh membrane and stain for b-actin. You should see a clear band minus the 250kDa.
I think it is the primary problem. Try antibodies from another company. Make sure they have a different immunogen (read the spec sheet) because some antibodies from different companies can be similar.
