how to seperate double-digestion products on agarose gel - (Sep/27/2011 )
After double digestion of my target plasmid, there are two products with similar sizes: one is 2.2 kb, the other is 2.3 kb. I have used 120 Voltage, 45 min, but these two bands were not be seperated with 1% agarose gel.
So I wonder is it possible they can be seperated?
Any suggestions are welcome!
You can try a different agarose type such as Nusieve with higher resolution and/or using a different buffer system (e.g. Lithium Borate instead of TAE). A longer gel also helps of course. Or use different enzymes so that you get fragments with a larger difference, if it's possible.
hobglobin on Tue Sep 27 20:04:31 2011 said:
You can try a different agarose type such as Nusieve with higher resolution and/or using a different buffer system (e.g. Lithium Borate instead of TAE). A longer gel also helps of course. Or use different enzymes so that you get fragments with a larger difference, if it's possible.
Thank you for your fast reply!
BTW, do you think it can be solved by increasing agarose concentation, like 2%? Becuase it will be more convinient.
Thanks!
You would be better off running it for longer rather than changing the % gel - 2% just means that they will migrate slower for bands of that size.
There may be an enzyme that will cut the the fragment you don't want.
phage434 on Tue Sep 27 23:47:29 2011 said:
There may be an enzyme that will cut the the fragment you don't want.
Thanks, it looks it is a good idea.
But I am wondering restriction enzyme can really 100% cut the fragment which I don't want, otherwise, this contaminatant will influence my futher ligation with another vector.
bob1 on Tue Sep 27 22:49:08 2011 said:
You would be better off running it for longer rather than changing the % gel - 2% just means that they will migrate slower for bands of that size.
I just tired to run with 1 hour at 130 voltage with 1% agarose, but these two bands still can not be seperated.
Attachment is the picture.
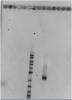
How about running at a lower voltage but overnight? I can usually get pretty good separation of larger bands for my RFLPs. I use 0.8% agarose in 0.5 TBE buffer and run overnight at 70V (or longer even), using a 23cm gel tray.
Another idea would be to design primers to amplify the region you want?
leelee on Wed Sep 28 15:11:07 2011 said:
How about running at a lower voltage but overnight? I can usually get pretty good separation of larger bands for my RFLPs. I use 0.8% agarose in 0.5 TBE buffer and run overnight at 70V (or longer even), using a 23cm gel tray.
It is a good idea!
I wonder how big your bands can be seperated in this case? My two fragments: one is 2.2kb, and the other is 2.3kb.
Thanks!
