MDCK cells vacuolation - Vacuolation of MDCK cells while staining (Oct/24/2009 )
Hi All,
I borrowed MDCK cells from my prof, and I started culturing them from passage 25 to passage 35, I am using them for viral studies.
When i stain the cells i see vacuolation. This is what I do,
Grow them on slides for 24-32 hours, wash them with PBS pH 7.2
Then fix them using 80% acetone or 80% methanol,
Then stain them using hematoxylin then eosin with steps involving dehydration, I see vacuolation even before dehydration using ethanol.
THis means that there is problem in the cells can someone helo me out with this problem, what should I do with the vacuolation?
I use DMEM as growth medium and have dissolved ampicillin.
Please help
Thanks
MS
Do you have any pictures? Are you sure it is vacuolation? Are the cells senescent?
yes i do have pics,
i will attach two of them, pls have a look. I will attach few more in sometime.
Please comment on them
MS

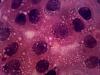

Your cultures are pretty confluent, it could be that the cells are senescent, though I have never worked with the line so I don't know if they are contact inhibited.
They aren't contact inhibited, but yours seem too confluent. They can form domes and can start to become polarised epithelial at high cell densities (if I remember correctly)
https://www.atcc.org/ATCCAdvancedCatalogSea...logy#aProp90e6a
the link above is to the ATCC which has a picture and their recommendations for culture conditions.
Do you have any from an lower passage number ?- you have got a fairly high passage number there.maybe try a "younger" cell and see if you have the same problems.
