ChIP form tissues - sonication problem? (Aug/18/2009 )
Hi,
I am about to start doing ChIP from endoscopic biopsies.
I fixed the chopped biopsies in 1% formaldehyde in medium for 20 min at RT.
Then I homogenized with bead grinder (precellys machine), 3 cycles 20sec 6000rpm (good homogenization).
Then I sonicated using Bioroptur for 8-12 cycles, 30sec on, 30 off (see attached gel).
Unfortunately, I am getting high MW DNA and do not know why...
could it be inefficient cell lysis ( i have used LB1,LB2,Lb3 according to R. Young protocol). When i did the same protocol for the cells, the sonication was fine.
any suggestions?
Thanks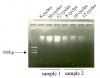
sushina on Aug 18 2009, 01:56 PM said:
I am about to start doing ChIP from endoscopic biopsies.
I fixed the chopped biopsies in 1% formaldehyde in medium for 20 min at RT.
Then I homogenized with bead grinder (precellys machine), 3 cycles 20sec 6000rpm (good homogenization).
Then I sonicated using Bioroptur for 8-12 cycles, 30sec on, 30 off (see attached gel).
Unfortunately, I am getting high MW DNA and do not know why...
could it be inefficient cell lysis ( i have used LB1,LB2,Lb3 according to R. Young protocol). When i did the same protocol for the cells, the sonication was fine.
any suggestions?
Thanks
In my hands I had to do more extensive sonication on my tissue samples than for cells. You might try diluting your lysates 2-fold and splitting them into two aliquots before sonicating.
Thanks for the reply.
I already tried diluting samples (3fold) without success.
Do u think I should increase sonication time (i saw people use up to 30/60 min).
which lysis buffer do u use for tissues and do u check the lysis under the microscope?
sushina on Aug 18 2009, 02:53 PM said:
I already tried diluting samples (3fold) without success.
Do u think I should increase sonication time (i saw people use up to 30/60 min).
which lysis buffer do u use for tissues and do u check the lysis under the microscope?
I use a lysis buffer containing 1% triton X and 0.5% NP-40 (so no SDS). I actually used the sonicator at low power to homogenize my samples before sonicating them at high power (mostly just to make sure they were homogeneous before splitting them into smaller aliquots).
Do you have access to a probe sonicator. I never tried using the Bioruptor for tissues and used a probe sonicator instead. Not because anyone I know had problems with using the Bioruptor on tissues. I just figured a probe sonicator would be more forgiving if the ratio of the amount of tissue to volume of buffer wasn't optimal.
Thanks for your reply. It sounds like a good advice.
Do you use low power sonication to complete disruption after a conventional
homogenization (grinder, Dounce, etc...) or to perform the tissue
homogenization itself? How many cycles/min would you recommend starting
with?
I notice you use 1%triton, whereas my lysis buffer (LB1) is only 0.25%. I'll
try increasing that as well. I kind of feel that my problem may be
incomplete cell lysis.
Thanks. Will let you know what transpires.
Hi Sushina,
Do you have access to a Covaris instrument? You can carryout the homogenization, and chromatin shearing in one step in the same tube.
Hamidk
