Protein not migrating through gel - (Jul/15/2015 )
I would really appreciate some help with this.
I am studying a membrane protein, around 40KDa but I am unable to detect the majority of the protein by SDS-PAGE as it seems to stay stuck at the top of the gel.
I have tried whole cell lysates (2x SDS+5%2-ME), NP-40 lysis buffer, RIPA buffer and dedicated membrane protein extraction kit but most of the protein refuses to migrate through the gel. I am over-expressing the protein by transfection and have tried both lipofectamine and Ca/P methods but these don't seem to be the cause.
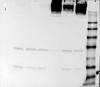
I have tried NuPage 10% Bis-Tris gel and Criterion TGX AnyKD gel. Both show the same thing (example attached).
I tend to run the gels at low voltage (around 100V) for 1-1.5h in general.
I have not tried using DTT yet.
Any ideas would be really appreciated.
how do you prepare your samples?
you may be overboiling.
do you use any reducing agent in your sample buffer?
1m cells (these ones are HEK293T cells) in 6 well plates. Wash twice with cold PBS. Scrape off in 1mL cold PBS into Eppendorfs. Spin twice and remove all PBS. Add 50uL 2x SDS gel loading solution (Quality Biologicals) containing 5% 2-ME. Vortex. Boil 95-99C for 5min. If it's still gloopy pipette up and down or vortex more. Sometimes store overnight at -80C and run gel next day. Usual loading of 15-20uL per well depending on how thick the sample is. If it's too thick, add more 2x SDS with 5% 2-ME to dilute.
you can try incubating at 60-70C for 10-20 minutes instead of 95-90C. that should eliminate the possibility of aggregated proteins.
you should ensure that the final concentration of sample buffer is 1X (and use 1X to dilute). the buffer should contain glycerol and will appear "thick" if not properly diluted.
"thick"ness is not a good determinator of protein concentration, especially if you want to match loading between wells.
dtt and 2-me in the same solution is redundant.
if you store the samples at any temperature lower than room temperature then you should reincubate to ensure that all sds crystals are resolubilized and that any aggregates that formed are broken up.
are you sure that a 10% gel covers the size range of interest. maybe you could perform some preliminary runs with a gradient gel. that could give you a handle on the sizes of the proteins that appear not to migrate well.
Thank you for all your suggestions. My apologies for the delay in replying - I was trying out your suggestions.
I tried heating the samples at both 70C and 60C for 8mins.
I also used 1x sample buffer both times, with only 5% 2-ME.
I ran both sets of samples on a gradient gel (the stack is 4% apparently).
As you can see form the attached, these measures gave no improvement. Almost all of the protein is still stuck at the top of the membrane. In fact, very little protein is now visible on the membrane.
Do you have any other suggestions?
Thank you in advance.
membrane proteins are notoriously difficult to work with. you should disrupt the membrane with ripa then perform a buffer exchange to remove the non-ionic surfactant(s) (they displace sds from the protein).
add the sds sample buffer with 2-me and incubate at 60-70C for 20 minutes (since it's a difficult sample).
if the protein still remains at the top of the gel then it's possible that it is a glycoprotein or lipoprotein. these post translational modifications can increase the apparent mass considerably. they will also make the bands "fuzzy".
you can stain the membrane (or gel) for glycoproteins to confirm.
Thanks. I took the lysates from yesterday (prepared with Ripa) and tried heating to 65C for 20 min vs 99C for 5 min. There was clearly more protein on the membrane at the size I was expecting, compared to stuck at the top, when heating to only 65C. But the majority was still stuck at the top (>95% of the protein I would estimate).
How about preparing lysates using a strong salt solution and no SDS instead? So, lyse in NaCl, HEPES, NaF, EDTA, NP-40 and protease inhibitors, take the supernatant, precipitate the protein using acetone and re-dissolve overnight in 1x SDS containing DTT, then heat at 65C for 20 min before loading on gel? Do you think this would work (and avoid buffer exchange)?
Thanks again.
precipitation and resuspension is buffer exchange.
it may work, no harm in trying.
but, if the protein is post-translationally modified (many membrane proteins are) then it shouldn't make a difference.
I'll try it and get back to you.
You might also consider isolating the membrane fraction by lysing in a hypotonic buffer (lower solute concentration in buffer than cells). Use mild mechanical disruption (syringe) and then use an appropriate centrifugation process to isolate your desired membrane fraction. Finally, try to solubilize your protein from the membrane pellet with a range of detergents/concentrations.
We've had good results with membrane proteins using this approach.