Ponceau on PVDF membranes affecting immunoprecipitation antibody staining?? - (Jun/25/2012 )
Hi,
So the Western I did today I was looking for a protein at around 115 kDa, I had already attempted to purify my protein from my lysate using Protein G coated beads and an antibody specific for my protein. I was looking for a phosphorylated response, so was then trying to stain with an anti-phospho-Tyrosine antibody.
After the transfer I blocked the membrane with 5% milk, then decided to do a ponceau stain to look for bands before proceeding (I know I know, doing ponceau after blocking is a silly idea as I have already found out while googling about this issue...). I did a Ponceau, (blocked, washed in PBS-T a few times, added the Ponceau for 4 mins, washed the membrane in water, saw the bands, then washed a few more times in PBS-T to get rid of the pink colour).
I then stained with my antibody (primary in 3-4% milk overnight at 4 degrees, secondary in milk 1H at room temp), only to find my bands were exceedingly faint, I had to expose for 15-20 minutes to get even these faint bands!
My question is regarding the faintness of the bands, could that be down to the Ponceau affecting the membrane? I did a similar experiment earlier int he week, using the exact same cell lysate (I had 100ul of lysate, used 50ul earlier and 50ul this time) and the exact same immunoprecipitation method each time. the only differences were the transfer time (the first time I didn't transfer long enough, and there were problems with the voltages as we had several blot tanks attached to the same powerpack) and this time I did a ponceau stain.
I am wondering whether my Ponceau protocol was wrong and affected the staining in a severe way, I know now that doing ponceau after blocking is a bad idea, and I have also read in some places online that you need to hydrate with methanol at some point in the ponceau method if doing it on PVDF membranes, but is this going to affect the entibody staining later?
Sorrry for the very long question but I wanted to include ll the information I could! I hope someone can help!
you may have to select a different blocking agent (eg-bsa). milk contains phosphoproteins (casein).
if the blot was faint, it may be due to preabsorption of the antibody by the blocking agent in the antibody solution.
I have never had much of a problem with milk background staining, and there isn't much background on the blot, so I don't think that is a problem, although I will block with BSA next time to see if it makes a difference.
I have attatched the image with the faint bands, you can see there is little background. This is after 20 minutes exposure!
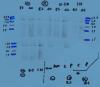
have you used this primary antibody before?
you may want to check both the primary and secondary antibodies (dot or slot blot with positive control and negative control for checking primary and dot or slot blot of primary to check secondary).
the primary could have been depleted by the milk block in the antibody solution. that would give low background and faint bands.
