smear restriction digest - (Feb/17/2012 )
Dear all
I having some trouble performing a restriction digest with BstBI and FseI on a plasmid. In attachment you can see the gel of the digest I ran:
lane 0: Mass Ruler
lane 1: uncut vector (about 500 ng)
lane 2: uncut vector (about 500 ng) at 37°C, 2h
lane 3: uncut vector (about 500 ng) with 1x BSA and 1x NEB buffer4, at 37°C, 2h
lane 4: vector (about 500 ng) cut with FseI (2 units), 1x BSA and 1 x NEB buffer4 at 37°C, 2h
lane 5: vector cut (about 500 ng) with BstBI (1 unit), 1 x NEB buffer4 at 65°C, 2h
lane 6: Mass Ruler
So, apparently when I add BSA and NEB buffer 4...my plasmid completely degrades! Both NEB buffer 4 and BSA were new and never used before. I also purified my plasmid using a High pure PCR purification kit of Roche to avoid contamination of DNAses...
Anyone can help me out...(My last option is to heat-schock my plasmid again and prep it very carefully)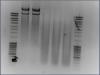
I would suggest that one of your reaction components has DNAses. This could be water, BSA, Buffer 4, or your plasmid. The Mg++ in the buffer 4 will act as a cofactor for the DNAse, so you might not see the problem unless buffer 4 is present, even though the DNAse is elsewhere.
